Balancing Net Ionic Redox Equations
For the reaction between NO 2 and H 2 the net charge on both sides of the equation in Step 1 is zero. This often occurs with hydrogen ions and water molecules in more complicated redox reactions.
Balancing Redox Equations 1.

. It winds up with the equation. Balance each of the following net ionic equations for redox reactions. The same goes for example balancing redox equations in.
5 A more detailed discussion about balancing this equation can be found here. An oxidationin which the atoms of one element lose electronsand a reductionin which the atoms of one element gain electrons. The following unbalanced net ionic equation provides an example.
Balancing net ionic redox equations The simplest way to express a redox reaction is an equation that shows only the oxidation and reduction processes. Solution for Balance the net ionic redox equations by the half-reaction method. Ive been working on this problem for about 30 minutes and Im still unable to achieve an answer.
Full reaction balanced in acid. Check that both sides of the equation have the same kind and number of atoms as well as the same charges. The corresponding ionic equation is.
Ionic chemical equations are slightly different from that of a classic case of chemical equations. Na a q Cl a q Ag a q NO 3 a q Na a q NO 3 a q AgCl s If you look carefully at the ionic equation. 6 I once saw an unusual method to balancing this particular example equation.
The balanced equation will be calculated along with the states complete ionic equation net ionic equation and spectator ions. To balance a redox equation using the oxidation state method we conceptually separate the overall reaction into two parts. Write balanced net ionic.
Balancing a Net Ionic Redox Equation step-by-step 1. Any help would be greatly appreciated. In order to understand how this is done consider the balanced equation for the reaction of ironII nitrate and.
Use uppercase for the first character in the element and. __CrO42 __C6H6 __Cr3 __CO2. This online quiz is intended to give you extra practice in concepts related to redox reactions including oxidation numbers identifying reducing and oxidizing agents writing net ionic.
Remember that a redox reaction must be balanced for the number of electrons. The net ionic equation is thus obtained. Finally for the specified chemical equation a window will pop.
Balance the following equations of redox reactions. Equation ref2021 is the net ionic equation for this reaction before. There are hydrogen ions on both sides which need to be simplified.
View Balancing Net Equations for Redox Reactionspdf from CHEMISTRY 031 at Malayan Colleges Laguna. ELECTROCHEMISTRY Electrochemistry BALANCING NET IONIC.
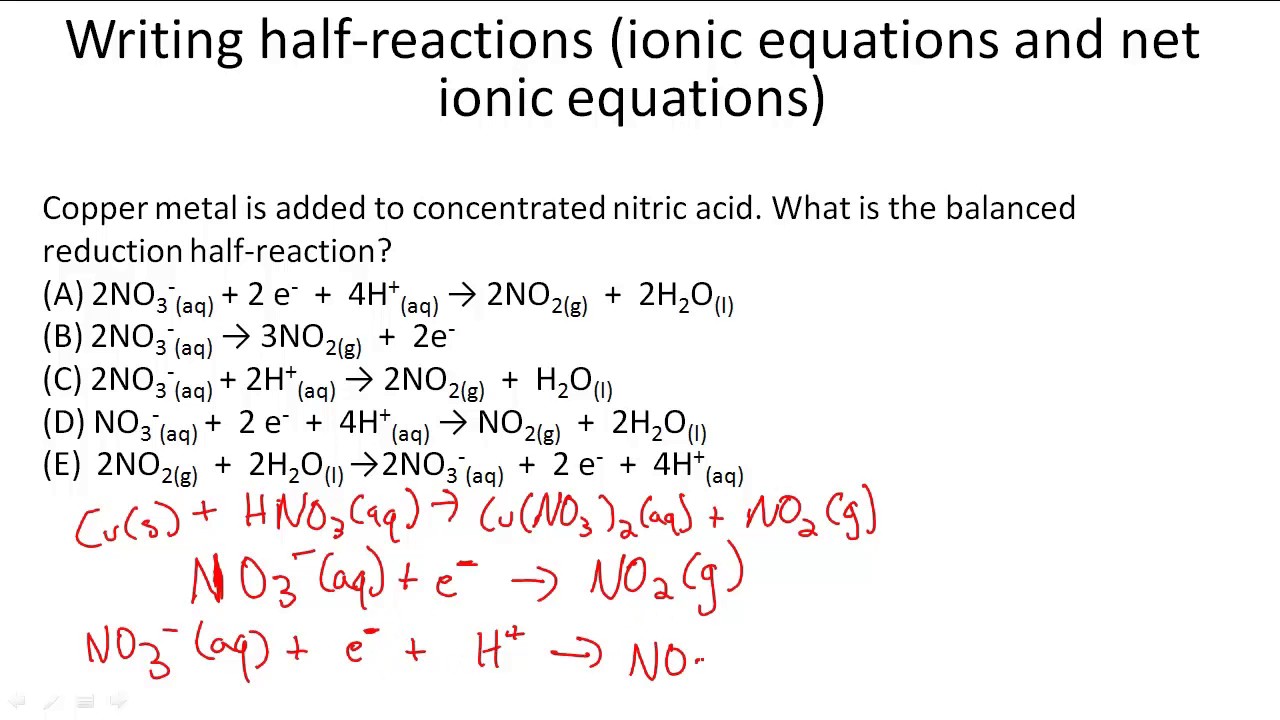
Writing Half Reactions Ionic Equations And Net Ionic Equations Youtube

Oxidation Reduction Redox Reactions Balancing Redox Reactions Chemistry Net Chemistry Lessons Teaching Chemistry Chemistry Classroom
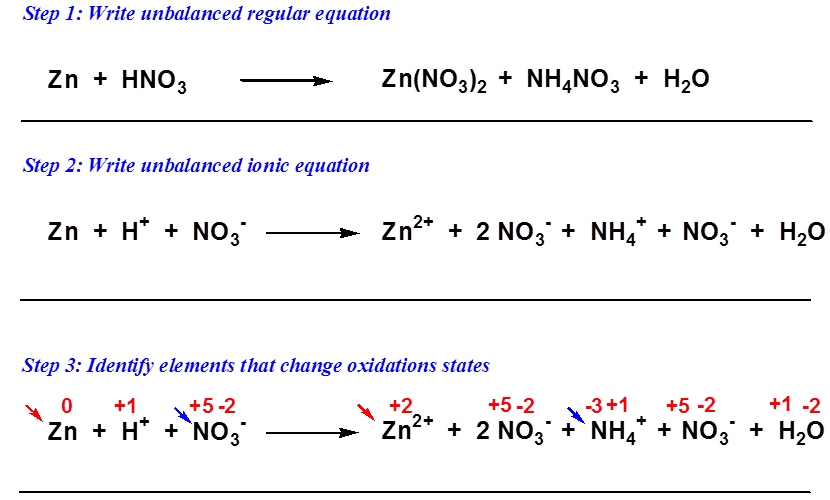

No comments for "Balancing Net Ionic Redox Equations"
Post a Comment